Impurities and Degradation products
On-Demand Synthesis of Drug Impurities
Oxidation Cyclisation, Rearrangement and more
Oxidation Cyclisation, Rearrangement and more:Manufacturers are responsible for controlling their processes and preventing the presence of undesirable impurities. They are also in charge to identify unacceptable impurities and develop methods for the measurement of amounts of impurities in drug substance and drug products. Organic impurities can arise during the manufacturing process and/or storage of the new drug substance.
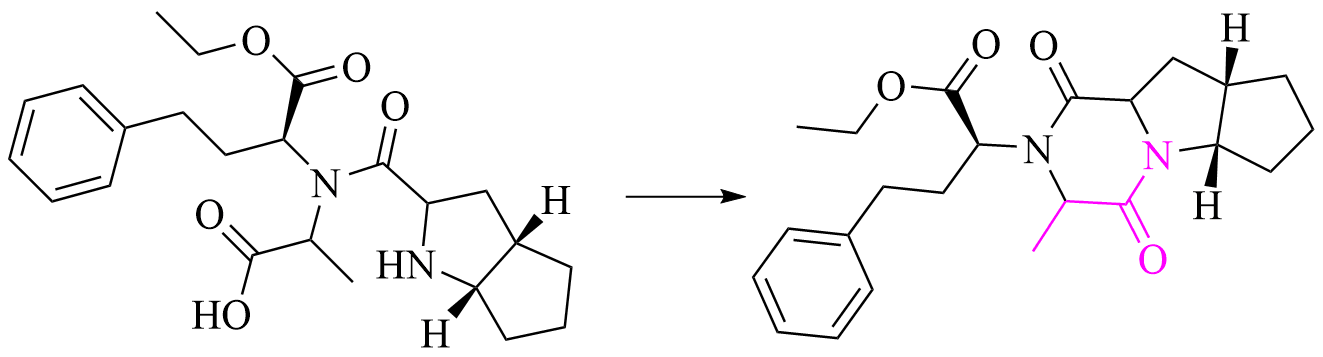
Ramipril Impurity
Ramipril EP Impurity D is an impurity standard of Ramipril. Intramolecular cyclization produced the diketopiperazine derivative.
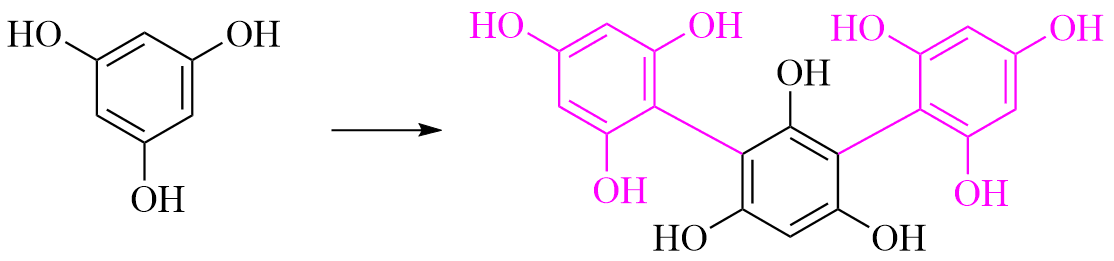
Phloroglucinol oxidation
Phloroglucinol is a polyphenol compound, it may me oxidized to Phlorotannins such as trifucol witch are thought to be marine-exclusive metabolites.
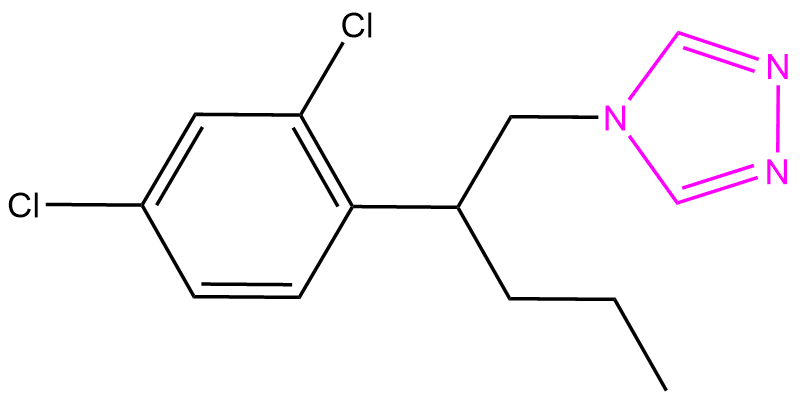
Penconazole impurity
Penconazole is an conazole based fungicide. Here is an example of triazole, positional isomer of Penconazole.
Please click and provide details to describe the custom product you are requesting.
Nitrosamine Custom Synthesis:
N-Nitroso (NOC) compounds have been studied extensively. They are of biological interest and many of their uses for the treatment of cardiovascular and central nervous system diseases, and diseases related to immunity and physiological disorders. While the body has mechanisms to detoxify some of these metabolites, the balance between activation and detoxification determines the risk associated with nitrosamine exposure. Nirosamine impurities can arise during the manufacturing process and/or storage of the new drug substance.
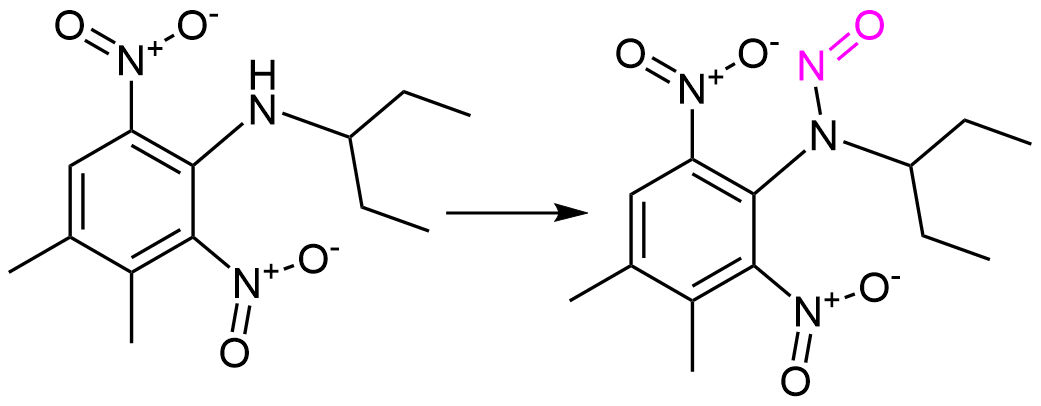
N-Nitroso Pendimethalin
Pendimethalin is used as a pesticide, its manufacture generally results in crude product with a N-nitroso-pendimethalin as impurity.
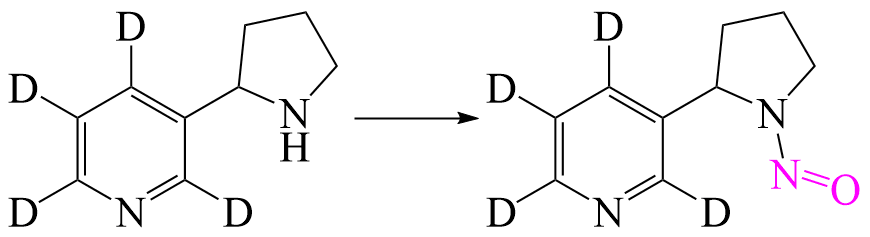
Labelled N-Nitroso Nornicotine
Air curing, burning and acidic conditions of the stomach degradate nicotine, and thus further the process of N-Nitrosation.
Please click and provide details to describe the custom product you are requesting.
Custom Synthesis services with competitive prices, fast delivery and high-quality products.
Stable Isotopes - Abundances %
The key elements in organic chemistry are carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), sulfur (S), chlorine (Cl), and bromine (Br). These elements naturally occur as mixtures of isotopes, which significantly influence the isotope distribution.
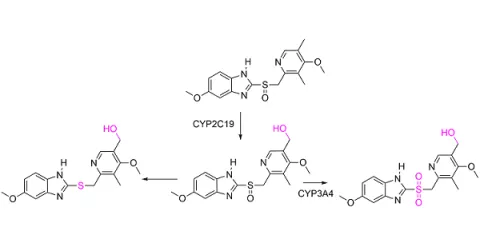
5-Hydroxy omeprazole standards
Omeprazole is primarily hydroxylated by CYP2C19 into 5-hydroxyomeprazole. 5-Hydroxy omeprazole retains the general structure of Omeprazole with an additional hydroxyl group on the pyridine ring, increasing its hydrophilicity.